In January, Columbia University revealed that four patients at its Irving Medical Center in New York had been sick with an unusual version of E. coli , a common gut bacterium. Although the news largely escaped attention in the media, it ricocheted through the world of infectious disease experts. E. coli is a relatively common bacterium and benign when it's in the gut, where it usually lives, but in the wrong places—such as in lettuce or ground beef, or our bloodstream—it can turn deadly. When antibiotics prove ineffective against an E. coli infection, as many as half the patients with it die within two weeks.
That's exactly why the Columbia E. coli was so worrying. Over the past decade or two, E. coli has developed resistance to one antibiotic after another. For some infected patients, their last hope is the antibiotic colistin, a toxic substance with potential side effects that include kidney and brain damage. The Columbia E. coli had a mutation in a gene, MCR-1, that confers a terrifying attribute: imperviousness to colistin.
"We're looking to the shelf for the next antibiotic, and there's nothing there," says Erica Shenoy, associate chief of the infection control unit at Massachusetts General Hospital. "We're facing the specter of patients with infections we can't treat."
Ever since an experimental miracle drug called penicillin was rushed to a Boston hospital in 1942 to save the lives of 13 victims of a nightclub fire, medical researchers have discovered more than 100 new antibiotics. We've needed each and every one of them—and they're not enough. It's not just E. coli . Drug-resistant strains of Staphylococcus , Enterobacteriaceae and Clostridium difficile have been steadily overcoming antibiotics; one study found that the number of deaths due to resistant infections quintupled between 2007 and 2015. Recently, treatment-resistant versions of the fungus Candida auris have shown up in hospitals in New York City and Chicago, killing half of infected patients.

The U.S. Centers for Disease Control and Prevention reports that 2 million people a year are sickened in the U.S. by bacteria or fungi resistant to major antibiotics, and that 23,000 die from them. "It's probably a vast underestimate," says Karen Hoffmann, who heads the Association for Professionals in Infection Control and Epidemiology. "We don't have a good reporting system for multiresistant organisms, so we don't really know." Studies suggest the cost to the U.S. health care system of treating patients with these hardy bugs tops $3 billion a year.

This grim trend is expected to accelerate. The World Health Organization predicts that worldwide death rates from drug-resistant microbes will climb from the current 700,000 per year to 10 million by 2050. At that point, they will have surpassed cancer, heart disease and diabetes to become the main cause of death in the human race. Before antibiotics, a small cut, tooth decay or routine surgery could lead to a life-threatening bacterial infection. Penicillin, the "miracle drug," and other antibiotics changed all that, saving countless lives over the years. But the age of the miracle drug seems to be ending.
Doctors are learning how to identify and isolate the bugs that are already resistant in the hopes of avoiding large outbreaks. They are scrambling to tighten up on the use of antibiotics in an effort to slow the development of resistant strains. It's too little, too late: The strategy will only buy us some time. At the moment, the oldest and weakest patients in hospitals are most affected, but the risks are spreading. "We're seeing healthy young people with urinary tract and skin infections that we don't have a pill for," says Helen Boucher, an infectious disease specialist at Tufts Medical Center in Boston. "And we may not be able to perform organ transplants, and even routine surgeries like joint replacements. We should all be scared."
Medical experts are pinning their hopes on entirely new strategies for dealing with infection. To find novel ways of killing bugs, they're looking in exotic places—in viruses and fish slime and even on other planets. They're using insights gained in genomics and other fields to come up with new technologies to kill bugs and keep them from spreading. And they are re-examining practices in hospitals and other spreading-grounds for bacteria, putting in place more holistic strategies for managing the bacteria in our bodies and in our hospitals and doctors' offices.
The alternatives sound promising, but they are far off. It's not clear that we can invent new weapons before the superbugs, like a zombie army at the gates, overwhelm our defenses.
"We need to make a huge investment in other approaches," says Margaret Riley, a drug-resistance researcher at the University of Massachusetts. "And we need to make it 15 years ago."
The New Bug-Hunters
Part of the problem with drug resistance is that microbes evolve with alarming speed into new species. Whereas a human needs 15 or more years to mature enough to have offspring, microbes like E. coli reproduce every 20 minutes. In a few years, they can go through evolutionary change that would have taken humankind millions of years to accomplish—change that can include acquiring genetic attributes that allow them to withstand drugs. A human on antibiotics is the perfect lab for developing resistant microbes. "Research shows that whenever a new antibiotic comes into use, we start to see the first resistant microbes emerge about a year later," says Mass General's Shenoy.
There's little in the pharmaceutical pipeline to replace the antibiotics to which bugs are becoming resistant. That's because development of a new antibiotic runs about $2 billion and takes about 10 years—with little hope of ending up with the sort of blockbuster drug that justifies such an investment. "The point of having a new antibiotic would be to use it as infrequently as possible, for as short a time as possible," says Jonathan Zenilman, chief of the division of infectious diseases at Johns Hopkins Bayview Medical Center in Baltimore. "Why would a pharma company want to develop a drug for a market like that?"
Medical researchers are now searching for other approaches. One involves recruiting biologists with a flair for evolutionary theory into the war on bugs. In the 1990s, Riley started out at Harvard and Yale studying the ways viruses kill bacteria and bacteria kill one another. In 2000, a colleague casually asked her if the work had any application to human health. "I had never thought about that," she says. "But suddenly everything clicked for me, and I became consumed by that question."
Riley has since spent the past two decades looking into applying the warfare strategy of viruses to the problem of resistant infections in humans. Viruses called "phages," which are basically chunks of genetic material wrapped in a protective protein, will pierce the cell wall of a bacterium and hijack its genetic machinery, turning the bacterium into a factory for making more viruses. Riley also studies how bacteria sometimes also kill other bacteria in the competition for food. A colony of bacteria will sometimes elbow out a competitor by producing poisonous proteins called "bacteriocins."
Riley's goal isn't just to kill dangerous bacteria—it's also to protect the beneficial ones. Of the roughly 400 trillion bacteria living in or on each of our bodies, the vast majority are helpful or benign—only one 10-thousandth of a percent of them are potentially harmful, she says. Commonly prescribed "broad spectrum" antibiotics like penicillin, ciprofloxacin and tetracycline don't discriminate between good and bad bacteria—they wipe out them all. That not only helps lead to the emergence of resistant bacteria but also causes problems for patients.
"An antibiotic is like throwing an H-bomb at an infection," Riley says. "You kill 50 percent or more of all the bacteria in the body, and a lack of healthy bacteria has been linked to obesity, depression, allergies and other problems." Phages and bacteriocins, on the other hand, can in theory be tuned to take out a colony of infection-causing bacteria in a patient, all without harming the normal flora or creating a fertile breeding ground for resistant bugs.
ImmuCell, a biotech company in Portland, Maine, has developed a bacteriocin that treats dairy cows for mastitis, a disease that costs the dairy industry $2 billion a year. Riley says labs like hers can adapt phages and bacteriocins to target virtually any sort of human microbial infection too, with little risk of nurturing new resistance. "These are stable, hardy killing mechanisms that evolved 2 billion years ago," she says.
Several clinical trials of phage therapy have already been successfully conducted in Poland, the nation of Georgia and Bangladesh. In the West, there have been successful phage trials for foot ulcers. No trials are underway for more serious infections, but a successful phage treatment of a critically multiresistant-infected patient in California in 2017 under Food and Drug Administration emergency rules has more researchers in the U.S. looking to develop phage treatments. One or more of these could move toward trials in the next few years, says Riley, including one for multi-resistant tuberculosis and another for pulmonary infections in cystic fibrosis patients. Bacteriocins are further behind. The U.S. government has promised to provide $2 billion for the effort to develop these alternatives, "but that's not nearly enough," she says.
Cancer researchers are widely investigating drugs that can boost immune systems, and these immunotherapies could be promising in helping weakened patients fight off resistant bugs that try to take hold. Researchers have produced human antibodies in cows and other animals that can be injected into patients. Boston's Harvard-affiliated Brigham and Women's Hospital, in an emergency effort, reported injecting a combination of antibodies and antibiotics to save a patient with a drug-resistant infection, but the results weren't disclosed. Otherwise, little has been done to bring the approach to trials in infected patients. Researchers are also working on vaccines against resistant staph infections and other resistant bacteria, but these too are just research efforts. "These non-antibiotic treatments are still in the early stages of investigation," says David Banach, who heads infection prevention at the UConn Health medical center in Farmington, Connecticut. "But we have to keep thinking of new approaches."
Given the enormous urgency of the problem, why is it taking so long to move promising solutions toward trials and availability? Because there's little money in it, says Tufts' Boucher. The government is sinking billions into research, but the private investment to turn that research into manufactured drugs and devices has not materialized. Drug companies, says Boucher, have little prospect of profiting off a drug that isn't likely to be taken by millions of people or fetch prices of tens of thousands of dollars per dose. "The economic model is broken," she says.
Managing the Bugs
Although antibiotics are truly miracle drugs when they work, our current problems have arisen in part because medicine has relied too heavily on them. Doctors prescribe them for ear infections, sore throats and urinary tract infections. Surgeons use them to prevent postoperative infections. Because bacteria can develop resistance, antibiotics make the most sense as part of a holistic approach to managing the spread of bacteria and dealing with infections. As antibiotics begin to lose their usefulness, medical experts are now coming around to emphasizing many-pronged strategies for keeping the bugs at bay.
Being quicker off the mark to identify and respond to potential outbreaks with extra precautions and targeted antibiotics could slow or prevent outbreaks. New tests under development would allow health workers to rapidly and cheaply identify the genes of any bacteria found on or near patients. "We can't do molecular screening on every patient who walks through the door for every organism. That would be looking for a needle in a haystack," says Shenoy. "But if we can screen high-risk patients quickly enough, we can take appropriate actions." It would certainly be an improvement over the standard techniques for identifying bacterial outbreaks, developed 150 years ago.
Infection specialists are also focusing on getting hospitals to do a better job at containing resistant bugs when they pop up, instead of spreading them among patient populations. About 5 percent of all patients in U.S. hospitals end up with a "nosocomial" infection—that is, an infection acquired at the hospital. It's not hard to see how. Hospitals are dense gatherings of sick people with weakened immune systems and various wounds and punctures, constantly poked and prodded by fingers and tools that move around the hospital to poke and prod others.
An aging population and new procedures have left patients all the more vulnerable. Johns Hopkins' Zenilman found in an informal survey he conducted that more than half of all the patients he asked had some sort of implant, a common source of infection. "The patients in hospitals today as a group are much sicker than any we've seen in history," he says. "Research shows that, on average, hospitals aren't following the right precautions about half the time," says the Association for Professionals in Infection Control and Epidemiology's Hoffmann. "It's our biggest struggle."
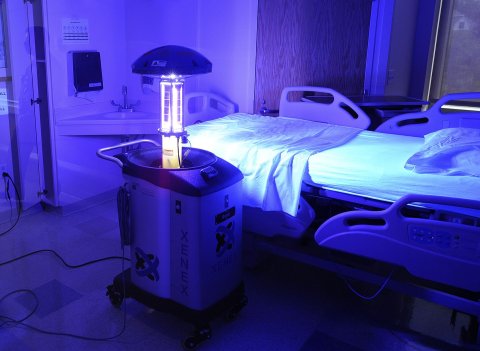
Hospitals are beginning to change their ways. Many now use trash can–shaped robots to disinfect walls with ultraviolet light (the rooms have to be empty because the light is harmful to humans too). At Riverside Medical Center, south of Chicago, two robots from a company called Xenex disinfect more than 30 rooms a day.
Keeping hospitals clean would be easier if bacteria couldn't stick to surfaces such as tabletops and clothes. Melissa Reynolds, a Colorado State University biomedical engineer, is developing new bacteria-resistant materials. Health care workers' clothing and other hospital materials and surfaces wouldn't need as much disinfection if they didn't pick up bugs in the first place. Fighting bacteria accidental mission for Reynolds. She had been investigating ways of avoiding clots in the meshes that surgeons use to keep patients' arteries open. Applying a coating of copper nanocrystals to the meshes seemed to keep blood cells from sticking to the surfaces. She noticed that bacteria didn't stick to the nanocrystal coatings either. Then a student in her lab had a eureka moment: Why not dip cotton fabric in a solution of the nanocrystals to keep bacteria from sticking to the fabric? "We were discovering new materials with strong antibiotic properties," says Reynolds. "That led us in a new direction."
The idea of bacteria-proof clothing has so far survived a battery of tests. "We've exposed the treated fabric to all kinds of bacteria over and over again, and we can't find any sticking to it," she says. "We're still trying to figure out the mechanism, but we know it works with all different types of bacteria." She has already collaborated with a major medical-supply company to prove that the nanocrystals can be cheaply incorporated into manufacturing processes. Now, she's investigating ways to get the crystals into a variety of other materials used in hospitals, including stainless steel, paint and plastics. These treated materials would stay bacteria-free much longer than conventional hospital surfaces that are wiped with common disinfectants, she says.
Lasers are another potential weapon against bacteria. Mohamed Seleem, a Purdue University biologist, and his colleagues were trying to come up with a way to quickly identify infectious bacteria in blood samples by hitting the samples with laser light of different colors. Along the way, they noticed that certain drug-resistant bacteria changed color from gold to white just seconds after being hit with a mild beam of blue laser light. Some of these "photobleached" bugs died, and others became so weakened that they lost their resistance to ordinary antibiotics. The blue light, it turned out, damages the pigment in the bacteria's outer membrane. "It only affects a particular pigment," says Seleem. "So it doesn't harm any other cells."

Seleem and his collaborators are now working to find ways to tune the color of the laser light to target other resistant bugs. If he succeeds, health care workers would be able to use a laser the size of a flashlight to safely kill dangerous bacteria on patients' skin or disinfect a hospital or doctor's office. It could also be shined on the skin and clothing of health care workers themselves in order to keep them from spreading infections. His collaborators are working to set up clinical trials.
Seleem also thinks the light could be used on severe, dangerously resistant blood infections, by hooking up a patient to a blood-circulating machine and shining it on the blood as it passes through. "You're basically taking out the patient's blood, sterilizing it and returning it to the patient," he says.
Slowing the Superbugs
Although the pharmaceutical industry has all but abandoned antibiotics, researchers haven't given up hope of finding new ones. The antibiotic revolution was kicked off in 1928, when Alexander Fleming returned from vacation to his London lab only to find a weird-looking mold growing in a dish he had left by an open window. Ever since, researchers have tried to peek into every corner of nature for the next great bacteria-killer. According to recent studies, among the new sources of substances that appear lethal to even resistant bacteria—yet may be safe for human consumption—are insects, seaweed, the mucus on young fish, arsenic-rich dirt in Ireland and even Martian soil. One team at Leiden University in the Netherlands is trying to build an artificial bacterium from scratch in hopes it can be tweaked to manufacture a new antibiotic.

Doctors are also trying to make the best use of antibiotics we have now by slowing the development of new resistant strains. That calls for cutting back on rampant overuse of antibiotics, which encourages superbugs to evolve. Doing so has to become an international effort, because the resistant bugs in one part of the world often made their way from elsewhere.
Developing countries are becoming a particularly frequent source for emergent bacterial threats that end up in the U.S., notes UConn's Banach. Studies have found that in most of the world antibiotics are readily dispensed from community pharmacies without prescriptions, contributing to a 65 percent climb in global antibiotic use between 2000 and 2015. The resulting resistant bugs skip frictionlessly around the world in the guts of millions of international travelers. "The impact of antibiotic overuse in these countries, as well as living and environmental conditions, are facilitating the worldwide spread of resistant organisms," he says.

Patients have a role to play too. Pressuring doctors to prescribe antibiotics for mild congestion, urinary tract infections or slow-to-heal wounds contributes to antibiotic overuse and the resulting resistance. Massachusetts public health officials have been pushing physicians and the public to ease up on antibiotics for more than a decade, and the state was rewarded with a 16 percent drop in prescriptions over a four-year period. A small victory, perhaps, in a large war that we have so far been mostly losing.
The cost of our failure to act a decade or more ago to this easily foreseeable crisis will most likely be a spreading tide of severe illness and death. It won't rise to the level of deadly mass outbreak the way a killer virus like Ebola could. But resistant infections will begin to affect more and more of us. Even if medicine, government and industry embarked today on a massive effort to find new approaches to combat resistant infections like the Columbia E. coli —which they aren't close to doing—the payoff wouldn't come for a decade or more.